Calibrating the HD-XG Continuous Glucose Telemetry Implant
Introduction
The HD-XG implant provides continuous measurements of blood glucose, temperature and activity in mice and rats. The glucose sensor is affected over the implant duration by the presence of fibrin, tissue and glucose levels. For optimal performance, the HD-XG must be calibrated using reference measurements over the course of a study:
- Initial multi-point calibration
- Twice weekly single-point calibration
- End-of-study multi-point calibration
Raw telemetry data are recorded in nanoamperes (nA) and calibration reference values are recorded in mg/dL or mmol/L. The calibration algorithm converts the telemetry data (nA) to values that are equivalent in the appropriate mg/dL or mmol/L units. The calibration process is most easily conducted by two people. Additional personnel can be added to streamline the process and increase throughput.
- First person: Records calibration values in the software system and directs the time of samples.
- Second person: Handles the animals, collects the blood samples at the times directed and reports the calibration values.
Selecting a Calibration Reference
Several calibration reference options exist, including glucose analyzers, reagents and diagnostic equipment, as well as glucometers with test strips. DSI recommends the Nova StatStrip® Xpress glucometer and test strips, as they provide comparable results to other laboratory analytics with the advantage and convenience of requiring smaller blood samples (1.2 uL) and providing immediate results.i The StatStrip Xpress provides measurement and correction for hematocrit and other common interferents and a higher level of accuracy than most alternative handheld glucometers. This document will refer to the use of a glucometer for collecting the reference measurements.
Multi-Point Calibrations
A multi-point calibration establishes a linear relationship between the sensor output and blood glucose levels. DSI recommends using two points (baseline and post-peak) for calibration purposes, but can support multiple points over the course of a glucose challenge. The blood glucose levels should differ by at least 200 mg/dL (11 mmol/L) to minimize calibration error caused by inaccuracies of the glucose reference. DSI recommends using an Oral Glucose Tolerance Test (OGTT) for multi-point calibration. An Intraperitoneal Glucose Tolerance Test (IPGTT) is also acceptable. Intravenous Glucose Tolerance Tests are not suitable for calibration.
Performing a multi-point calibration:
- View the continuous glucose data by having appropriate graphs setup in Ponemah (see the Ponemah User Manual for a Glucose Calibration Tutorial which details how to set up graphs and enter calibration values).
- Draw duplicate blood samples while the study animal has a steady baseline glucose value.
- Record baseline calibration values from the glucometer. The acquisition computer time to the nearest 5 seconds and corresponding glucose value are required. Using the Glucose Calibration window in Ponemah ensures correct time alignment of reference samples. Alternatively, the calibration values and associated times can be recorded in a spreadsheet.
- Increase the animal’s blood glucose level with an oral or intraperitoneal glucose administration. For normal, healthy rats, administering 3-6 g dextrose/kg will increase the blood glucose level by at least 200 mg/dL (11 mmol/L).
- Observe the telemetry glucose data. When the glucose level peaks note the time and prepare to draw and record duplicate blood samples again in 3-5 minutes .
- This 3-5 minute post peak measurement is desired as it minimizes error associated with lag between central and peripheral glucose. Essentially it is desirable to obtain the post peak measurement at a time when glucose has been stable or has changed minimally in the last 3 to 5 minutes.
Table 1: Sample data table for a multi-point calibration using an OGTT. Dual samples were taken for all reference measurements.
|
SAMPLE |
Baseline Time |
Test Strip Measurements (mg/dL) |
Elevated Time |
Test Strip Measurements (mg/dL) |
|
Animal #1 |
10:03:10 |
101, 105 |
10:23:30 |
307, 313 |
|
Animal #2 |
10:04:20 |
99, 108 |
10:24:20 |
315, 305 |
|
Animal #3 |
10:06:00 |
102, 97 |
10:26:40 |
303, 297 |
Single Point Calibrations
Single-point calibrations help account for non-physiologic changes in the baseline glucose value over time. Examples of non-physiologic changes include sensor drift due to enzyme instability or fibrin and tissue growth. Single point calibrations should be performed at least twice per week at the same time of day and during a time period when the animal’s blood glucose is relatively stable.
Taking a single point calibration:
- Draw duplicate blood samples while the animal has a steady baseline glucose value.
- Record baseline calibration values from the glucometer. The acquisition computer time to the nearest 5 seconds and corresponding glucose value are required. Using the Glucose Calibration window in Ponemah ensures correct time alignment of reference samples. Alternatively, the calibration values and associated times can be recorded in a spreadsheet.
Best Practices
Leaving Telemetry Device ON During the Entire Study
Leave the HD-XG implant in ON mode throughout the entire study to improve glucose sensor stability and longevity. When the device is off the enzymatic reaction still occurs and a product builds up on the sensing electrode which may damage it. Turning the device ON after extended time in OFF mode will result in a positive spike and it will take 1-5 hours for the glucose values to normalize. If an implant is turned OFF mid-study, a single-point calibration should be performed at least 5 hours after turning ON. A multipoint calibration would be preferred.
Take Duplicate Blood Samples for Each Reference Value
- Duplicate samples should be used to minimize error and establish the most reliable calibration of the implantable glucose sensor. If duplicate samples vary by >10%, one or more additional samples are recommended to establish a more accurate reference value.
- Take duplicate glucose samples from a single point in time by drawing blood from the animal and testing the blood glucose level twice (e.g. using two different test strips). Enter the two reference values in the software at the same time point and the software will average them.
Observe Telemetry Data During Multi-point Calibration
In a normal rodent, the baseline blood glucose level might be approximately 100 mg/dL (5.5 mmol/L), while the peak value might be 300 mg/dL (16.7 mmol/L) after an OGTT has been initiated. Peak glucose values will typically occur 12-16 minutes post-dose during an OGTT, but may vary from animal to animal. Observing the real-time dose response will ensure a stable blood glucose level has been reached and provide the most accurate calibration possible. Observe the real-time dose response through having appropriate graphs setup in Ponemah. If telemetry data cannot be viewed in real-time during calibration, a study animal’s response to OGTT should be characterized prior to the first multi-point calibration to estimate the appropriate postdose time for the peak blood glucose sample.
Minimizing Stress, Anesthesia Artifacts
- Taking blood samples too frequently from animals that are stressed (due to restraint) can cause significant bias and variability in reference samples.
- Taking samples from anesthetized animals is discouraged as isoflurane may impact the glucose sensor reading, particularly at later points in the study period.
Considerations & Alternatives
In order to optimize implant calibration, there are several factors to consider.
- The number of calibration points to use. Two points in time are sufficient for multi-point calibration as long as they are taken during stable baseline and peak glucose levels that differ by at least 200 mg/dL (11 mmol/L). Using more calibration points can help average out error, but it is difficult to compensate for error due to lag between the central and peripheral glucose levels when samples are taken on the rising or falling edge of the glucose curve. Taking calibration points too frequently can also introduce significant stress on the animal and variability in the measurements, particularly when blood is drawn from the tip of the tail.
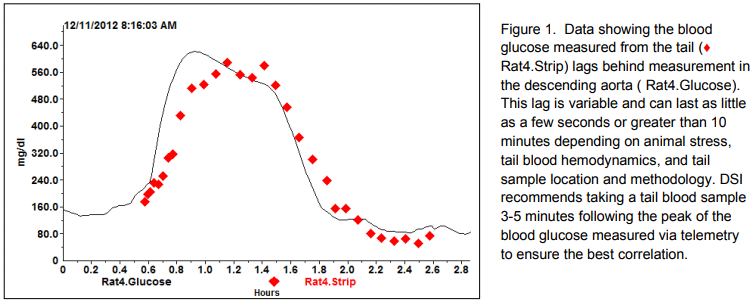
- Potential lag time between the glucose values taken by the implanted sensor and the calibration reference. In a normal, healthy animal the peak glucose value is typically observed 4-12 minutes post dose for an Intraperitoneal Glucose Tolerance Test (IPGTT) and 12-16 minutes post dose for an OGTT. These durations will vary based on the glucose dose, whether or not the animal was fasted and the animal model. A blood sample taken from the tip of an animal’s tail may have a 2-5 minute (or more) delayed response to the glucose dose due to stress artifact and the hemodynamics of the tail. Figure 1 demonstrates the glucose measurement lag between the descending abdominal aorta and tail of a rat. The tail sample has a peak glucose value that occurs later than the sensor in the descending aorta detects a peak value, which could contribute error in the calibration process. By sampling several minutes after the peak is observed in the telemetry signal, the stable periods for the implant and reference signal can be more closely aligned and the theoretical calibration error can be reduced.
- Alternate methods for blood sampling. A method for minimizing the impact of lag between the central and peripheral blood is to sample from blood that is more central. As an example the saphenous vein rather than the tip of the tail. Such sampling would not change our recommendation for timing of samples but would lessen the impact that lag has. In mice it is recommended that saphenous vein sampling be written into your protocols as an allowed method. Doing so provides an approved and alternative blood sampling method should the need arise (e.g. inability to get a blood sample from the tail and/or the measure from the tail appearing blatantly incorrect).
- Alternate methods for increasing blood glucose levels. Several methods can be used to increase blood glucose levels to at least 200 mg/dL (11 mmol/L). Oral (OGTT) or IP glucose tolerance tests (IPGTT) can be used and the method chosen depends on your study needs. IPGTTs typically result in faster and higher glucose peaks than OGTTs. IPGTTs can expedite the calibration process and aid in achieving the desired glucose difference of 200 mg/dL (11 mmol/L), however, glucose is metabolized more quickly and the peak glucose value lasts for a shorter period of time. OGTTs require a larger bolus of glucose to achieve the target 200 mg/dL (11 mmol/L) difference. However, peak glucose levels typically remain stable for a longer period of time, resulting in an easier and more accurate calibration process.
When using Type 1 or Type 2 diabetic animals, an Insulin Tolerance Test (ITT) can be substituted for the glucose tolerance test, with the baseline value becoming the higher value.
If using an IPGTT, Insulin Tolerance Test (ITT) or other method, please consult with DSI on the proper timing of the second sample. IVGTTs are generally not recommended as the sensor cannot respond fast enough.
i “Assessment of Nova Biomedical StatStrip® Glucose Meters and Test Strips in Rodent Glucose Studies.” Richard G. Peterson, Robert Brockway. Poster 1127.11, Experimental Biology Meeting, San Diego, CA, April 2012.
Comments
0 comments
Please sign in to leave a comment.