Calibration process to perform RC Studies on anesthetized Large Animals
In order to perform RC studies on anesthetized large animals, you need to have the RC site type to obtain the proper signal and analyze/derive RC data.
In addition, the appropriate sized endotracheal tube for the animal will need to be sourced, as well as an oral gavage (esophageal) tube.
DSI does not supply these. They may be purchased from the following vendors.

Calibration Process
When using a QT1001, calibration is a manual process. After a hardware configuration is created, the calibration tool runs you through a series of steps to calibrate.
Flow Calibration
Flow calibration requires injecting a known volume of air though the pneumotach with a large syringe while the flow transducer is connected to it.
It is recommended that the pneumotach heater be turned on and warmed up prior to performing the flow calibration.
Pressure Calibration
For pressure calibration, it requires creating a 2-point calibration by applying 0 cmH2O (low) and X cmH2O (high) to the pressure transducer. Traditional methods use a water manometer that allows for displacement.
The standard Buxco calibrator is designed for 20 cmH2O displacement. It may be possible to use this for the high calibration, should this displacement be enough for your species.
In either scenario, you will use:
- Water manometer/calibrator
- Tubing
- 3-way Luer stopcock
- Syringe
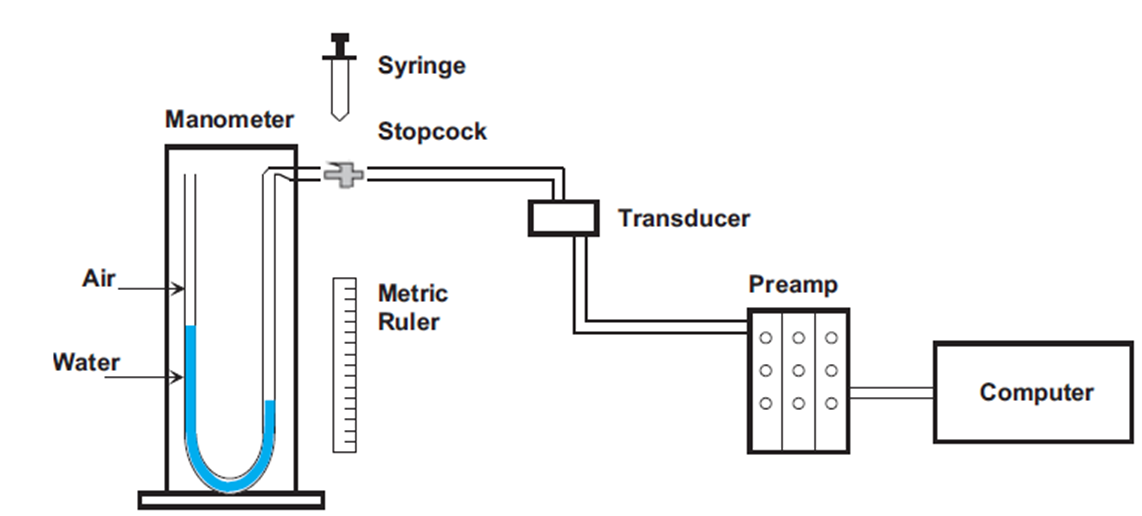
The pressure transducer should remain in a fixed position during calibration and data collection.
Prior to calibration, set up the manometer, syringe, stopcock, and transducer. Also, ensure all connections to the preamplifier and computer are established.
To perform the low calibration, ensure that there is no pressure (zero pressure) applied to the manometer and the transducer. You can even have the system open to atmosphere if you wish. Follow the calibration instructions in FinePointe.
To perform the high calibration, pre-load the syringe with air. Attach the syringe to the stopcock and ensure that the transducer connection (tubing connected to the stopcock) is closed. This means that if you were to inject air, it would only be applied to the manometer.
Next, push air from the syringe to the manometer until displacement reaches X cmH20 and is table.
Then, you turn the stopcock so the syringe is closed and the transducer is then open to manometer.
The X cmH2O is then reflected on the transducer and can be calibrated in FinePointe. It should be a stable signal. Once stable, you can click the “Next” button to finish calibration and see the effective ranges.
Instrumenting the anesthetized animal
- An endotracheal tube is placed in the anesthetized animal. With dogs and monkeys, use the largest bore endotracheal tube that is anatomically feasible to place in the trachea and attach the endotracheal tube to the pneumotachograph. The pneumotachograph should be attached to a flow pressure transducer connected to the amplifier.
- A pressure cannula is placed in the esophagus and attached to a pressure transducer. The pressure transducer should be connected to the amplifier. When you begin data acquisition and prior to marking baseline, check the position of the pressure cannula so as to achieve maximum pressure deflection with minimum amount of cardiac artifact.
Comments
0 comments
Please sign in to leave a comment.